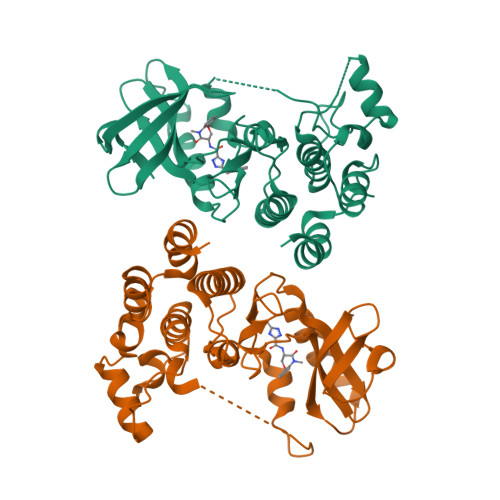
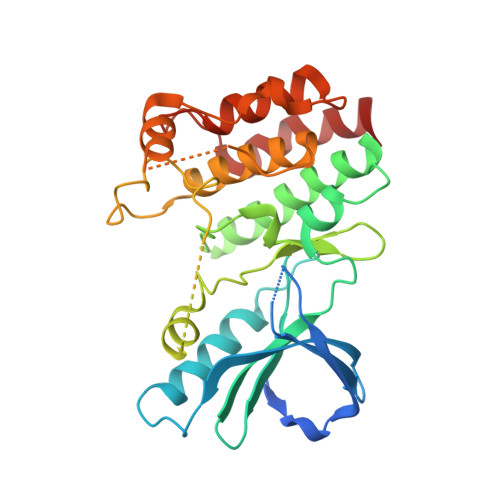
Discovery of a First-in-Class Receptor Interacting Protein 1 (RIP1) Kinase Specific Clinical Candidate (GSK2982772) for the Treatment of Inflammatory Diseases.
Harris, P.A., Berger, S.B., Jeong, J.U., Nagilla, R., Bandyopadhyay, D., Campobasso, N., Capriotti, C.A., Cox, J.A., Dare, L., Dong, X., Eidam, P.M., Finger, J.N., Hoffman, S.J., Kang, J., Kasparcova, V., King, B.W., Lehr, R., Lan, Y., Leister, L.K., Lich, J.D., MacDonald, T.T., Miller, N.A., Ouellette, M.T., Pao, C.S., Rahman, A., Reilly, M.A., Rendina, A.R., Rivera, E.J., Schaeffer, M.C., Sehon, C.A., Singhaus, R.R., Sun, H.H., Swift, B.A., Totoritis, R.D., Vossenkamper, A., Ward, P., Wisnoski, D.D., Zhang, D., Marquis, R.W., Gough, P.J., Bertin, J.(2017) J Med Chem 60: 1247-1261
- PubMed: 28151659
- DOI: https://doi.org/10.1021/acs.jmedchem.6b01751
- Primary Citation of Related Structures:
5TX5 - PubMed Abstract:
RIP1 regulates necroptosis and inflammation and may play an important role in contributing to a variety of human pathologies, including immune-mediated inflammatory diseases. Small-molecule inhibitors of RIP1 kinase that are suitable for advancement into the clinic have yet to be described. Herein, we report our lead optimization of a benzoxazepinone hit from a DNA-encoded library and the discovery and profile of clinical candidate GSK2982772 (compound 5), currently in phase 2a clinical studies for psoriasis, rheumatoid arthritis, and ulcerative colitis. Compound 5 potently binds to RIP1 with exquisite kinase specificity and has excellent activity in blocking many TNF-dependent cellular responses. Highlighting its potential as a novel anti-inflammatory agent, the inhibitor was also able to reduce spontaneous production of cytokines from human ulcerative colitis explants. The highly favorable physicochemical and ADMET properties of 5, combined with high potency, led to a predicted low oral dose in humans.
Organizational Affiliation:
Centre for Immunobiology, Blizard Institute, Barts, and The London School of Medicine and Dentistry, Queen Mary University of London , E1 2AD London, U.K.